Main Second Level Navigation
Breadcrumbs
- Home
- Research
- Programs of Research
- Episodic Disability and Rehabilitation Research Program
- Episodic Disability Questionnaire (EDQ) Study
Episodic Disability Questionnaire (EDQ) Study

Background
Disability is an important health-related outcome to consider as more individuals are aging with chronic conditions. The HIV Disability Questionnaire (HDQ) is a patient-reported outcome measure (PROM), originally developed to measure the presence, severity and episodic nature of disability among adults living with HIV. The 69-item HDQ includes six domains: physical, cognitive, mental-emotional symptoms and impairments, uncertainty and worrying about the future, difficulties with day-to-day activities, and challenges to social inclusion. We developed a short-form version of the HIV Disability Questionnaire (SF-HDQ) to facilitate use in clinical and community-based practice among adults living with HIV.
What do we mean by Disability?
In an earlier phase of research, people living with HIV defined disability as any physical, cognitive, mental or emotional health symptoms and impairments, difficulties carrying out day-to-day activities, challenges to social inclusion and uncertainty (or worrying about the future) (O’Brien et al, 2008; O’Brien et al, 2009).
Health related challenges (or disability) has been defined by some people living with persistent (or long-term) health conditions as any challenge because of a health condition. These challenges can be in six areas:
- Physical health challenges
- Cognitive health challenges
- Mental and emotional health challenges
- Uncertainty or worry about the future
- Difficulties with day-to-day activities
- Challenges taking part in social and community life (social inclusion)
Disability can be experienced as episodic in nature where health challenges fluctuate on a daily basis or over the longer course living with a health condition. Measuring disability is important for determining the impact of health conditions and for identifying interventions that may reduce health challenges.

What is the aim of the Episodic Disability Questionnaire?
The Episodic Disability Questionnaire (EDQ) is a self-administered questionnaire with the aim to describe the presence, severity and episodic nature of disability experienced by people living with chronic health conditions. The EDQ was derived from the Short-Form HIV Disability Questionnaire (SF-HDQ) that was established from the original Long-Form (original) HIV Disability Questionnaire (HDQ).
Evolution of the Episodic Disability Questionnaire (EDQ)
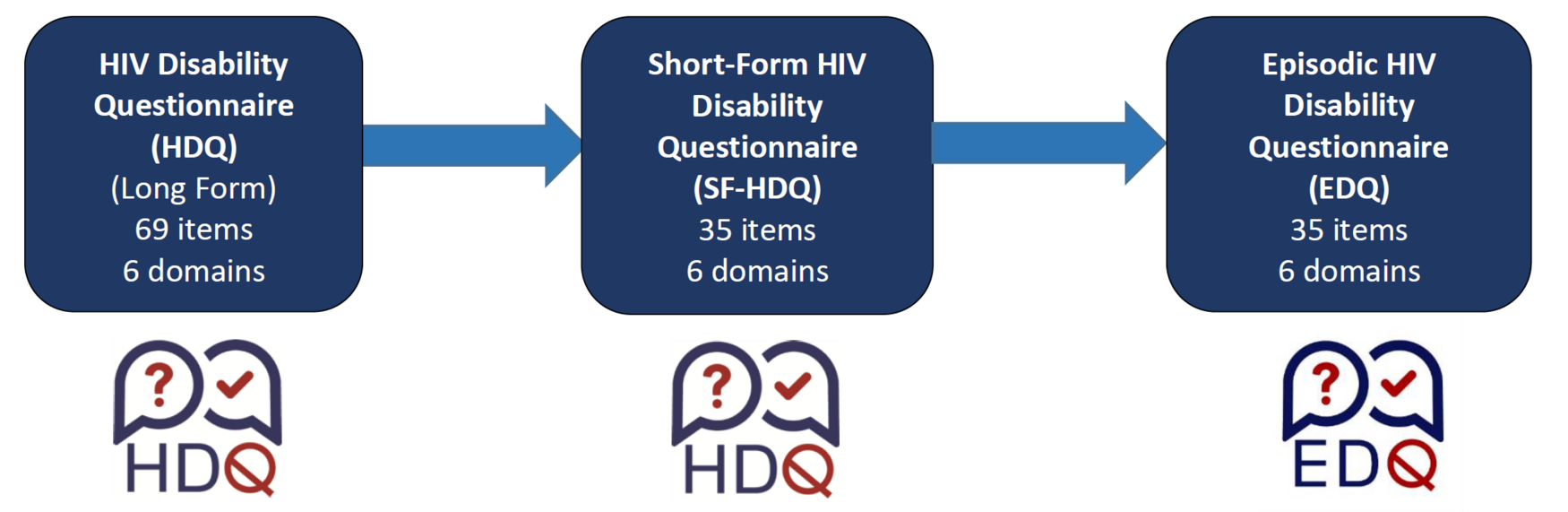
Long-Form (Original) HIV Disability Questionnaire (HDQ)
The HDQ is comprised of 69 items across six domains: physical symptoms and impairments (20 items), cognitive symptoms and impairments (3 items), mental and emotional health symptoms and impairments (11 items), uncertainty (14 items), difficulty with day-to-day activities (9 items) and challenges to social inclusion (11 items). Each item consists of a statement about a health-related challenge and has both a five point ordinal response scale asking the respondent to rate the challenge on the day of administration (from 0 to 4) and a nominal response scale asking whether the challenge fluctuated (or changed) over the past week (‘Yes’ or ‘No’). An additional item asks individuals to classify their health as having a ‘good day’ or ‘bad day’ living with HIV. The HDQ takes about 11 minutes to complete.
Item Generation
The items in the HDQ were derived from the Episodic Disability Framework, a conceptual framework of disability derived from the perspectives of adults living with HIV in Canada (O’Brien et al, 2008; O’Brien et al, 2009).
Measurement Properties
The HDQ demonstrates sensibility, internal consistency, reliability, construct validity and test-retest reliability when administered to samples of community-dwelling adults living with HIV in Canada, Ireland, United States, and the United Kingdom.
How to access the HDQ? The HDQ is under a Creative Commons Licence. To request the HDQ for use, please visit: https://research.mcmaster.ca/industry-and-investors/hiv-disability-questionnaire-hdq/.
Short-Form HIV Disability Questionnaire (SF-HDQ)
The SF-HDQ is comprised of 35 items across six domains: physical symptoms and impairments (10 items), cognitive symptoms and impairments (3 items), mental and emotional health symptoms and impairments (5 items), uncertainty (5 items), difficulty with day-to-day activities (5 items) and challenges to social inclusion (7 items). Each item consists of a statement about a health-related challenge and has both a five point ordinal response scale asking the respondent to rate the challenge on the day of administration (from 0 to 4) and a nominal response scale asking whether the challenge fluctuated (or changed) over the past week (‘Yes’ or ‘No’). An additional item asks individuals to classify their health as having a ‘good day’ or ‘bad day’ living with HIV. The HDQ takes about 3 minutes to complete.
The SF-HDQ is scored based on the Rasch for severity and presence scores. For more information on the SF-HDQ and how it was developed using Rasch with the Long Form HDQ: https://hqlo.biomedcentral.com/articles/10.1186/s12955-020-01643-2
Measurement Properties
The SF-HDQ demonstrates sensibility and utility for use with adults living with HIV in Canada, Ireland, United States, and the United Kingdom.
Episodic Disability Questionnaire (EDQ)
The EDQ is a generic disability questionnaire comprised of the same 35 items across six domains in the SF-HDQ: physical symptoms and impairments (10 items), cognitive symptoms and impairments (3 items), mental and emotional health symptoms and impairments (5 items), uncertainty (5 items), difficulty with day-to-day activities (5 items) and challenges to social inclusion (7 items). The SF-HDQ is scored based on the Rasch for severity and presence scores.
What are the next steps for the EDQ?
We are assessing the measurement properties of the EDQ for use in clinical and community-based practice with adults living with HIV (HIV in Motion; NIH study), and with adults living with Long COVID (CIHR Long COVID Study).
How can I get a summary of the research study results?
Updates on the EDQ study and publications will be openly available on the CIHRRC website (http://cihrrc.ca).
Publications
Click here to see them.
- Vader K, Chan Carusone S, Aubry R, Ahluwalia P, Murray C, Baxter L, Robinson G, Ibanez-Carrasco F, Stewart A, Solomon P, O’Brien KK. Examining the Utility of the HIV Disability Questionnaire (HDQ) in Clinical Practice: Perspectives of People Living with HIV and Healthcare Providers. Journal of the International Association of Providers of AIDS Care (JIAPAC). February 17, 2022. Vol. 21. https://doi.org/10.1177%2F23259582221079148
- O'Brien KK, Dzingina M, Harding R, Gao W, Namisango E, Avery L, Davis AM. Developing a short-form version of the HIV Disability Questionnaire (SF-HDQ) for use in clinical practice: a Rasch analysis. Health Qual Life Outcomes 2021; 19, 6. https://doi.org/10.1186/s12955-020-01643-2. Available at: https://hqlo.biomedcentral.com/articles/10.1186/s12955-020-01643-2
- O’Brien KK, Kietrys D, Galantino ML, Parrot JS, Davis T, Tran Q, Aubry R, Solomon P. Reliability and Validity of the HIV Disability Questionnaire (HDQ) with Adults Living with HIV in the United States. Journal of the International Association of Providers of AIDS Care. (JIAPAC). 2019. Volume 18. Jan-Dec;18:2325958219888461. Available at: https://doi.org/10.1177/2325958219888461
- Brown DA, Simmons B, Boffito M, Aubry R, Nwokolo N, Harding R, O’Brien KK. Evaluation of the psychometric properties of the HIV Disability Questionnaire among adults living with HIV in the United Kingdom: A cross-sectional self-report measurement study. PLoS One. 2019; 14(7): e0213222. https://doi.org/10.1371/journal.pone.0213222.
- O’Brien KK, Solomon P, Bergin C, O’Dea S, Iku N, Stratford P, Bayoumi AM. Reliability and validity of a new HIV-specific questionnaire with adults living with HIV in Canada and Ireland: the HIV Disability Questionnaire (HDQ). Health and Quality of Life Outcomes. 2015. 13:124.
- O’Brien KK, Bayoumi AM, Bereket T, Swinton M, Alexander R, King K, Solomon P. Sensibility Assessment of the HIV Disability Questionnaire. Disability and Rehabilitation. 2013 Apr;35(7):566-77. doi: 10.3109/09638288.2012.702848. Epub 2012 Jul 21.
- O`Brien KK, Bayoumi AM, Stratford P, Solomon P. Which dimensions of disability severity does the HIV Disability Questionnaire (HDQ) measure? An exploratory factor analysis. Disability and Rehabilitation. 2015;37(13):1193-1201. doi: 10.3109/09638288.2014.949358. Epub 2014 Aug 13.
- O’Brien KK, Solomon P, Bayoumi AM. Measuring Disability Experienced by Adults Living with HIV: Assessing Construct Validity of the HIV Disability Questionnaire using Confirmatory Factor Analysis. BMJ Open. September 1, 2014. 2014;4:e005456 doi:10.1136/bmjopen-2014-005456 https://bmjopen.bmj.com/content/4/8/e005456
- O’Brien KK, Bayoumi AM, King K, Alexander, R, Solomon P. Community Engagement in Health Status Instrument Development: Experience with the HIV Disability Questionnaire. Progress in Community Health Partnerships: Research, Education and Action. Winter 2014: 8(4): 549-559
People
Researchers
- Kelly O’Brien, Department of Physical Therapy, University of Toronto,
- Patty Solomon, McMaster University, Hamilton, Ontario, Canada
- Ahmed Bayoumi, St. Michael’s Hospital & University of Toronto, Toronto, Ontario, Canada
- Colm Bergin, Trinity College Dublin, St. James’s Hospital, Dublin, Ireland
- Richard Harding, King’s College London, London, United Kingdom
- Aileen Davis, University of Toronto
- Kristine Erlandson, University of Colorado Denver
- Soo Chan Carusone, McMaster University, Hamilton, Ontario, Canada
- Kristine Erlandson, University Colorado Denver, Denver, Colorado, United States
- Steven Hanna, Health Research Methods, Evidence, and Impact, McMaster, University.
- Jaime Vera, Brighton and Sussex University Hospital NHS Foundation Trust, Brighton, United Kingdom
- Carolann Murray, Casey House, Toronto, Ontario, Canada
- Lisa Avery, Dalla Lana School of Public Health, University of Toronto
- Darren Brown, Chelsea and Westminster Hospital, NHS Foundation Trust, London, United Kingdom
- Marta Boffito, Chelsea and Westminster Hospital, NHS Foundation Trust, London, United Kingdom
Coordinator Team (NIH Study)
- Kiera McDuff, PT, Research Coordinator, University of Toronto
- Noreen O’Shea, PT, St. James’s Hospital, Dublin, Ireland
- Mallory Boyd, Research Coordinator, University of Colorado Denver
- Natalie St. Clair-Sullivan, PT, Brighton and Sussex University Hospital NHS Foundation Trust, Brighton, United Kingdom
Funding
The HDQ development study was funded by a grant from the Canadian Institutes of Health Research (CIHR), HIV/AIDS Research Program and a Michael DeGroote Postdoctoral Fellowship (McMaster University). The SF-HDQ / EDQ development study was funded by the National Institute On Aging of the National Institutes of Health (NIH) (Award Number R21AG062380).
Kelly O’Brien is supported by a Canada Research Chair in Episodic Disability and Rehabilitation.
For More Information:
Contact Kelly O’Brien (Principal Investigator) at kelly.obrien@utoronto.ca